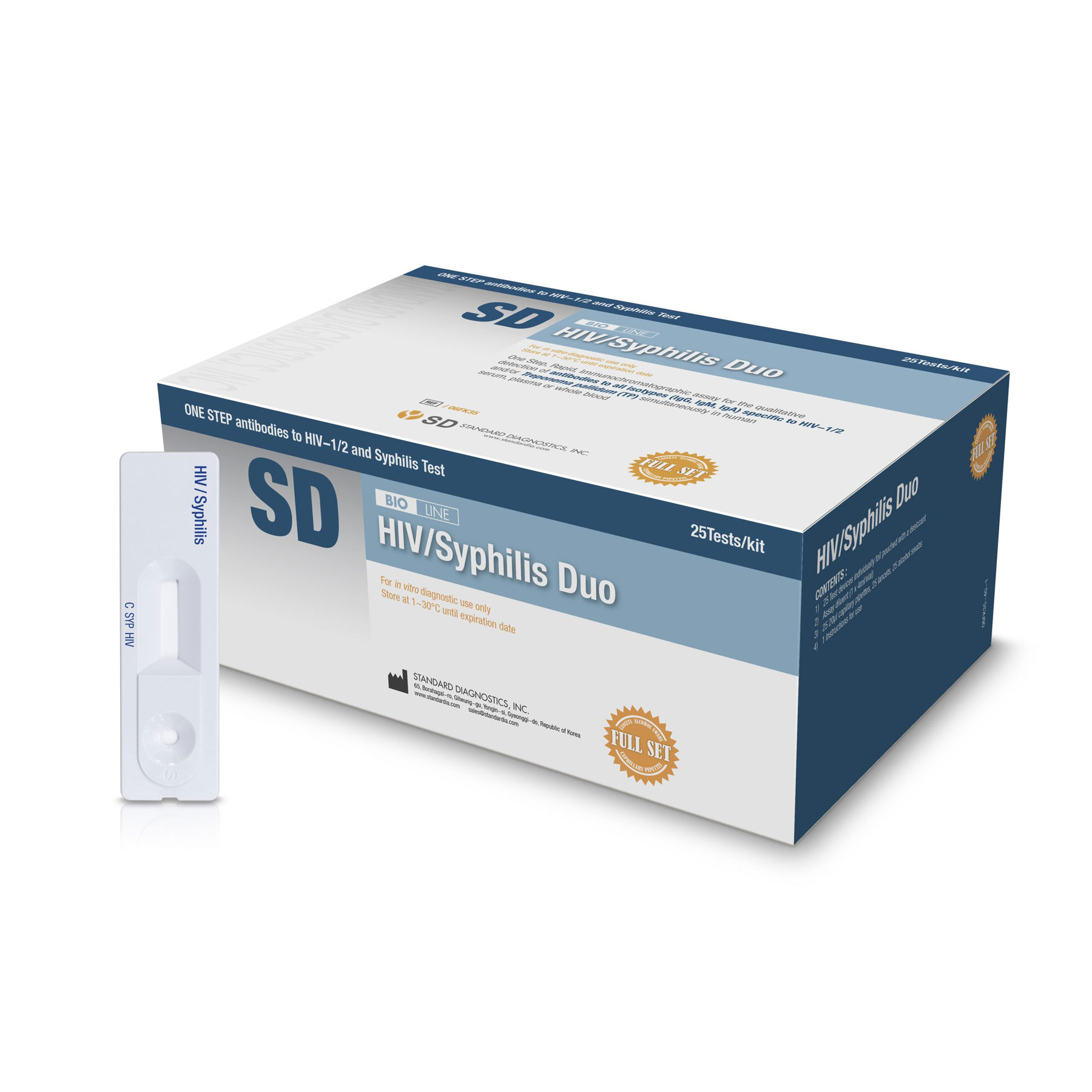
Product Information
Rp 665.297
SAME DAY TEST AND TREATMENT TO MAXIMIZE YOUR PMTCT PROGRAM.
The SD BIOLINE HIV/Syphilis Duo test is a solid phase immunochromatographic assay for the qualitative detection of antibodies to all isotypes(IgG, IgM, and IgA) specific to HIV-1/2 and/or Treponema pallidum (TP) simultaneously in human serum, plasma, or whole blood.


THE CHALLENGES
- No ability to test and treat same day
- Requires electricity, instruments, laboratory equipment etc.
- HIV cannot be tested at the same time
- Waiting to take two tests in different sites
- Collecting blood twice
- Patient has to revisit hospital for results
- Heavy workload
- Hard to access antenatal clinic (ANC) services: walking >1 hour
BURDEN OF SYPHILIS IN ASIA
0.24%
Prevalence of maternal syphilis as 0.24% for the Western Pacific Region
0.32%
Prevalence of maternal syphilis as 0.32% for the South-East Asia Region
167,000
CASES
maternal syphilis have occurred
6,800
ADVERSE OUTCOMES
including early fetal deaths

PATIENTS
- Convenience of one sample
- Reduces time for testing
- Same day test & treatment
- Enables immediate partner notification
CLINICIANS
- Reduces workload
- Reduces training time
- Single procedure, two results
PROGRAM MANAGERS
- No instruments, electricity or laboratory equipment required
- Increasing test coverage
- Cost-effective by reducing cost of storage, logistics, local distribution
- Contributes to SDG
PERFORMANCE
DETECTION OF HIV ANTIBODIES
DETECTION OF TREPONEMA PALLIDUM ANTIBODIES
| Country | Sensitivity Estimate (exact 95% CI) | Specificity Estimate (exact 95% CI) | Sensitivity Estimate (exact 95% CI) | Specificity Estimate (exact 95% CI) |
|---|---|---|---|---|
| Ghana | 100% (98.54%, 100%) | 100% (97.57%, 100%) | 100% (98.54%, 100%) | 99.33% (96.34%, 99.98%) |
| Togo | 100% (98.20%, 100%) | 100% (96.61%, 100%) | 100% (95.89%, 100%) | 99.35% (96.41%, 99.98%) |
| Myanmar | 100% (96.82%, 100%) | 99.24% (95.82%, 99.98%) | 98.67% (92.79%, 99.97%) | 99.20% (95.62%, 99.98%) |
| Kenya | 99.71% (98.40%, 99.99%) | 100% (98.96%, 100%) | 100% (95.75%, 100%) | 100% (99.40%, 100%) |
| Mexico | 100% (97.69%, 100%) | 100% (99.01%, 100%) | 99.07% (94.90%, 99.98%) | 99.67% (98.20%, 99.99%) |
| Laos | 100% (93.28%, 100%) | 97.09% (91.72%, 99.40%) | 100% (54.07%, 100%) | 100% (96.38%, 100%) |
| TOTAL | 99.91% (99.51%, 100%) | 99.67% (99.16%, 99.91%) | 99.67% (98.82%, 99.96%) | 99.72% (99.29%, 99.92%) |
COST EFFECTIVENESS
SUMMARY RESULTS FROM THE COHORT DECISION MODEL COMPARING THE EXPECTED EFFECTS (DISABILITY-ADJUSTED LIFE-YEARS (DALYS)) OF THE PREGNANCY AND COSTS PER DALY (2012 U.S. DOLLARS) FOR HIV AND SYPHILIS TESTING ALGORITHMS
| Total Costs | Cost increase from no program | DALYs | DALYs prevented compared to no program | CER comparing screening to no program | ICER* | |
|---|---|---|---|---|---|---|
| No program | $20,783,454 | – | 269,400 | – | – | – |
| Dual HIV/syphilis | $21,274,678 | $491,224 | 228,829 | 40,571 | 12.11 | 12.11 |
| HIV test only | $21,583,611 | $800,158 | 235,716 | 33,684 | 23.75 | Strictly** dominated |
| Single rapid tests for HIV & syphilis | $21,593,145 | $809,692 | 235,023 | 34,377 | 23.55 | Strictly** dominated |
| HIV rapid test & RPR/TPPA for syphilis | $21,605,356 | $821,902 | 235,094 | 34,306 | 23.96 | Strictly** dominated |
* Incremental cost effectiveness ratio (ICER) is the ratio of the change in costs to incremental benefit of an algorithm ** If an algorithm is both more costly and less effective than another algorithm it is considered “strictly dominated” and would not be recommended
| IA : Induced abortion | DALY : Disability-adjusted life years | CER : Cost-effectiveness ratio | ICER : Incremental cost-effectiveness ratio |
ACCEPTANCE STUDY
Background (Maternity Hospital and Ventanilla-Callao, Peru)
- This study was carried out to evaluate the
acceptability among ANC service providers - There were problems using separate HIV and
Syphilis rapid tests from technicians:
• Confusion with reagent and results
• Stock outs of HIV test - D BIOLINE HIV/Syphilis Duo tests were provided to 10 ANC health centers
Results
- 100% reported satisfaction with SD BIOLINE HIV/Syphilis Duo test
- 100% considered the SD BIOLINE HIV/Syphilis Duo
test to be better than separate tests - 32% mentioned difficulties with reading results (now printed ‘HIV’ and ‘SYP’ below the test lines
ACCEPTANCE SURVEY
Adapted from: Acceptance of HIV/Syphilis Duo Rapid Testing: Survey of Attitudes of Antenatal Care Providers in Peru
MAXIMIZE
PMTCT SERVICE WITH
HIV+SYPHILIS DUO
SIMPLE | RAPID | ACCURATE

RESULTS IN 15-20 MINUTES

SERUM, PLASMA, WHOLE BLOOD

1-30°C 24 MONTHS

ORDER INFORMATION
| Cat. No. | 06FK30 | 06FK35 |
|---|---|---|
| Specimen | S / P / WB | S / P / WB |
| Type | Device | Device |
| Pack Size | 25T/Kit | 25T/Kit |
| Dimension | 177 x 110 x 75 mm | 177 x 110 x 75 mm |
| Weight | 250G | 300G |
| Contents | Devices, Diluent | Devices, Diluent, Capillary Pipettes, Alcohol Swabs, Lancets |
| Carton | 565 x 415 x 395mm (55Kits) | 565 x 415 x 395mm (55Kits) |
| Certification | CE Marked (06FK30CE, 06FK35CE) | |
References
- Regional framework for the triple elimination of mother-to-child transmission of HIV, hepatitis B and syphilis in Asia and the Pacific, 2018–2030. Manila, Philippines, World Health Organization Regional Office for the Western Pacific. 2018. Licence: CC BY-NC-SA 3.0 IGO
- Bristow, C.C. et al. (2014) Multisite Laboratory Evaluation of a Dual Human Immunodeficiency Virus (HIV)/Syphilis Point-of-Care Rapid Test for Simultaneous Detection of HIV and Syphilis Infection. Open Forum Infect Dis. 1(1).
- UCLA Program in Global Health, Klausner, J. (2014) Dual rapid tests are costeffective to prevent mother-to-child transmission of HIV infection, syphilis and adverse pregnancy outcomes. [Onilne] Available at: http://www.dualelimination.org/#!cost-effectiveness/cnqx Accessed 28 Aug 2015.
- Acceptance of HIV/Syphilis Duo Rapid Testing: Survey of Attitudes of Antenatal Care Providers in Peru by Maria Valderrama MSH. [Online] https://cdc.confex.com/cdc/std2014/webprogram/Paper34834.html
| Weight | 0,2 kg |
|---|
| No | Spesifikasi | Product |
|---|---|---|
| 1 | Nama Produk | SD BIOLINE HIV/Syphilis Duo |
| 2 | Merk | SD BIOLINE |
| 3 | Pabrik | STANDARD DIAGNOSTICS, INC |
| 4 | Negara Asal | Korea Selatan |
| 5 | Prinsip Pemeriksaan | Immunochromatography Test |
| 6 | Kemasan | 1 boks terdiri atas;
|
| 7 | Kemampuan Deteksi |
|
| 8 | Hasil Test | Kualitatif |
| 9 | Spesimen | Whole blood, Serum, Plasma |
| 10 | Volume Sampel | 10 µl untuk serum atau plasma dan 20 µl untuk whole blood |
| 11 | Sensitivitas & Spesifisitas | HIV (Sensitivitas 100% dan Spesifisitas 100%) Syphilis (Sensitivitas 100% dan Spesifisitas 99,1%) |
| 12 | Suhu Penyimpanan | 2-30ºC |
| 13 | Kadaluarsa | 24 bulan dari tanggal produksi |
| 14 | No Katalog | 06FK30 |
| 15 | Registrasi | Terdaftar di Kementrian Kesehatan RI |
| 16 | No Registrasi | AKL 30305515934 |
Rp 450.000


Rp 50.000



Reviews
There are no reviews yet.